Proteins can be reversibly phosphorylated – phosphate groups are added to proteins by the action of kinase enzymes and can be removed by phosphatases. This controls a huge variety of cellular processes and targeting phosphorylation thus offers a board range of therapeutic opportunities. Indeed, kinases have received much attention in pharmaceutical research, yet phosphatases have been largely untapped. Anne Bertolotti’s group in the LMB’s Neurobiology Division has previously identified inhibitors, Guanabenz and Sephin1, that can selectively target a specific holophosphatase, R15A-PP1. When given to mice, Sephin1 prevents two protein misfolding diseases. New work by the group has now uncovered the mechanism underlying this inhibition, opening up the possibility to target other holophosphatases and treat disease.
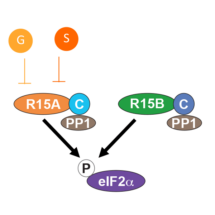
Activity of the PP1 phosphatase relies on regulatory subunits, which recruit specific protein substrates to the enzyme for dephosphorylation. There are over 200 different regulatory subunits that can combine with PP1 to form holophosphatases which dephosphorylate a wide range of proteins in cells. Two such regulatory subunits are R15A and R15B. Both bring the same substrate protein, eIF2a, to the PP1 phosphatase, but only R15A is inhibited by Guanabenz and Sephin1. Marta Carrara from Anne’s group created an assay using only three recombinant proteins – the PP1 catalytic subunit, the R15 regulatory subunit and the eIF2a substrate – and used the assay to uncover how Guanabenz and Sephin1 act, inhibiting R15A but not R15B.
To test whether Guanabenz and Sephin1 cause R15A to change in shape, Marta measured the sensitivity of R15 to enzymes which can break it into pieces. Addition of Guanabenz or Sephin1 reduced the sensitivity of R15A to this breakdown, but had no effect on R15B. This strongly suggests that Guanabenz and Sephin1 selectively cause a conformational change of R15A. Next, by splitting R15 into two parts, Marta showed that the C-terminal end of R15 binds to PP1 whilst the N-terminal binds to the eIF2a substrate. Adding Guanabenz or Sephin1 reduced the binding of R15A to eIF2a but had no effect on R15B. Finally, swapping the N- and C-terminals of R15A and R15B made Guanabenz and Sephin1 inhibit the new R15B but not the new R15A. Thus, Guanabenz and Sephin1 inhibit PP1-R15A by interfering with the ability of the N-terminal of R15A to recruit the eIF2a substrate, likely by altering the conformation of R15A.
This is the first study to directly elucidate the role of regulatory subunits in the function and selectivity of holophosphatases. Sephin1 has shown impressive activity against two neurodegenerative diseases and understanding the mechanism of action is an important step forward in future drug development. This work demonstrates that regulatory subunits of phosphatases are valid drug targets and provides the knowledge at the molecular level to expand this concept to other holophosphatases. There are more than 200 such phosphatases in humans that could in principle be studied using the system developed here, opening up a broad range of possibilities to investigate a large class of potential drug targets.
This work was funded by the Medical Research Council and the European Research Council.
Further references:
Paper in Nature Structural & Molecular Biology
Anne’s group page
Previous Insight on Research article: First step in preventing neurodegenerative diseases