Filaments in frontotemporal lobar degeneration with TDP-43 pathology Type C unexpectedly include annexin A11, which assembles alongside TDP-43 in a unique heteromeric structure
Neurodegenerative diseases are characterised by the accumulation of amyloid filaments in the brain. These filaments, which centre around specific proteins and adopt diverse structures depending on the disease, have a causal role in inciting neurodegeneration. Therefore, in order to develop new diagnostic and therapeutic interventions, it is vital to have a comprehensive knowledge of the structural, molecular and cellular mechanisms of filament formation. Using electron cryo-microscopy (cryo-EM), the structures of filaments from several diseases have been determined to date, all of which have been homomeric amyloid filaments characterised by the self-assembly of a single protein. Benjamin Ryskeldi-Falcon’s group in the LMB’s Neurobiology Division, have now determined filament structures from the brains of individuals with the neurodegenerative disease frontotemporal lobar degeneration with TDP-43 pathology (FTLD-TDP) Type C, revealing a heteromeric structure with two core proteins for the first time.
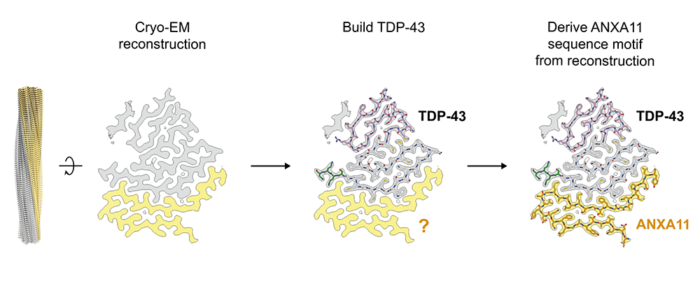
After isolating filaments from the brains of four individuals with frontotemporal lobal degeneration Type C, first author Diana Arseni used cryo-EM to determine their structures. The high resolution of the structures enabled the direct identification of the filament’s protein constituents (see Figure above). This led to the unexpected discovery of a second protein, annexin A11 (ANXA11), which co-assembles with TDP-43 to form heteromeric amyloid filaments. Prior to this finding, ANXA11 was not known to form amyloid filaments in neurodegenerative diseases, nor to play a role in FTLD-TDP Type C.
Diana collaborated with the groups of Masato Hasegawa at the Tokyo Metropolitan Institute of Medical Science, Japan, Bernardino Ghetti at the Indiana University School of Medicine, USA, and Marsel Mesulam at the Feinberg School of Medicine, USA, who provided brain samples and carried out neuropathological examinations. Their findings underlie the importance of using donated human tissue samples to unravel the biological mysteries behind common diseases.
The study adds ANXA11 to a small group of proteins known to form disease-associated amyloid filaments, alongside tau, TDP-43, α-synuclein, TAF15 and amyloid-β. The unprecedented evidence of heteromeric amyloid filament formation in the human brain presents an entirely new avenue of exploration in regards to understanding amyloid assembly, and may prove significant to the pathogenesis of neurodegenerative diseases. Finally, the research offers new insights into frontotemporal dementia by revealing the structures behind FTLD-TDP Type C, which is one of the most common causes of sporadic frontotemporal dementia. By defining the molecular pathology of this form of FTLD, this study identifies a target for the future development of new diagnostic and therapeutic tools.
This work was funded by UKRI MRC, the Hans Und Ilse Breuer Stiftung, the US National Institutes of Health, the Japan Agency for Medical Research and Development, the Japan Science and Technology Agency, the Japan Society for the Promotion of Science, the Tokyo Metropolitan Institute for Geriatrics and Gerontology, and the Leverhulme Trust.
Further references
Heteromeric amyloid filaments of ANXA11 and TDP-43 in FTLD-TDP Type C. Arseni, A., Nonaka, T., Jacobsen, M.H., Murzin, A.G., Cracco, L., Peak-Chew, S.Y., Garringer, H.J., Kawakami, I., Suzuki, H., Onaya, M., Saito, Y., Murayama, S., Geula, C., Vidal, R., Newell, K.L., Mesulam, M., Ghetti, B., Hasegawa, M., Ryskeldi-Falcon, B. Nature
Benjamin’s group page
Diana’s group page
Masato Hasegawa, Tokyo Metropolitan Institute of Medical Science, Japan
Bernardino Ghetti, Indiana University School of Medicine, USA
Marsel Mesulam, Feinberg School of Medicine, USA
Previous Insight on Research articles
Discovery of a new amyloid-forming protein in neurodegenerative disease
TDP-43 forms amyloid filaments with distinct folds in different neurodegenerative conditions
Structural breakthrough in study of aggregated TDP-43 protein in brains of ALS and FTD sufferers