Study using human kidney organoids finds that PAX8 is critical for driving renal morphogenesis
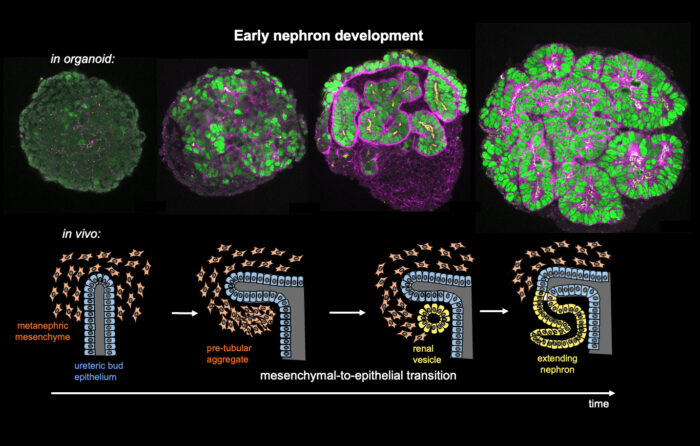
It is well established that organ shape is critical for organ function, as defects in the embryonic development of organs can lead to diseases such as spina bifida or polycystic kidney disease. However, how organ shape (and therefore organ function) is encoded by the genome is poorly understood. Now, Katja Röper and John-Poul Ng-Blichfeldt in the LMB’s Cell Biology Division, in collaboration with Julie Williams from AstraZeneca have studied human kidney development using renal organoids grown from human induced stem cells to better understand kidney morphogenesis.
At the earliest stages of kidney development, cells undergo a transition from unpolarised mesenchymal precursor cells to sheets of epithelial cells which can then transform into differentiated 3D nephron tubes. By the time they are born, humans will have up to a million nephron tubes in their kidneys, forming the functional units that filter unwanted substances and water out of the blood in the form of urine and return useful substances back into the bloodstream.
Using single cell RNA sequencing and analysis of chromatin accessibility to map early kidney development in renal organoids, the group have uncovered a novel nephrogenic transcriptional programme which drives the human mesenchymal-to-epithelial transition (MET). John-Poul conducted experiments using CRISPR to restrict gene expression. They found that the transcription factor PAX8 plays a key role in driving the polarisation to a renal vesicle, and without it, the crucial first step in renal morphogenesis fails and no nephron tube is formed. PAX8 fulfils this role by activating a cell-cell adhesion programme, which is an important part of the epithelial polarisation process. Additionally, while it is known that Wnt/β-Catenin signalling specifies nephron fate, the team found that it must be attenuated to allow other transcription factors to drive completion of MET. The team propose that there is an intrinsic mechanism to limit this signalling.
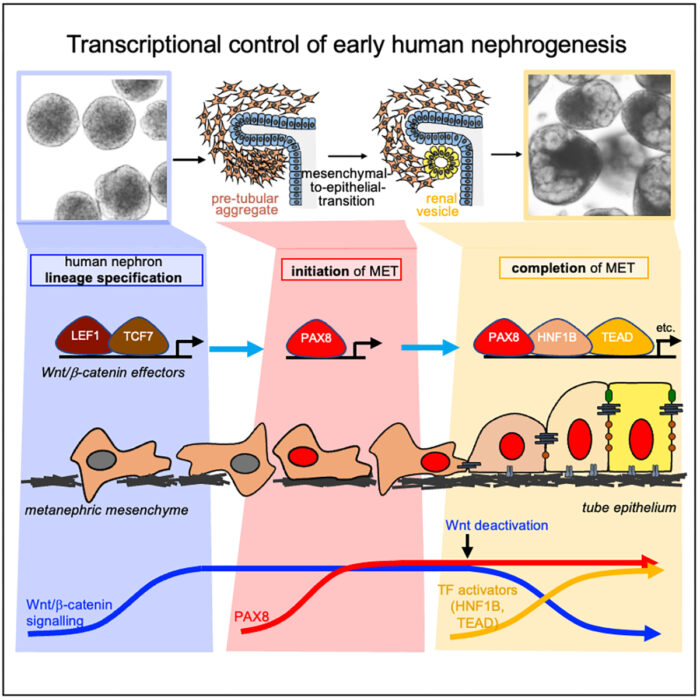
Interestingly, in vivo studies on mice kidneys did not find that PAX8 was crucial for initiation of MET. This, coupled with other recent comparative studies of mouse and human foetal kidneys, highlight the significant differences between mouse and human nephrogenesis and therefore the benefit to conducting studies using human renal organoids.
The researchers corroborated these findings and their organoid model by collaborating with Benjamin Stewart and Menna Clatworthy, based at the University of Cambridge’s Molecular Immunity Unit (housed at the LMB), to compare their RNA-sequencing data from human organoids with a sequencing dataset of human foetal kidney and found that the organoid system recapitulated human foetal changes to a large degree.
This study’s findings further our knowledge of human renal development, and developmental METs in general, as well as the kidney’s response to injury and repair. Around 10% of the global population is affected by chronic kidney disease, and a better understanding of nephrogenic developmental pathways could lead to new therapeutic approaches that target repair pathways. Finally, insight into the mechanisms of human MET also holds clinical significance given the emerging role of MET in distant metastatic colonisation in human cancer progression.
This work was funded by UKRI MRC, NIHR, the Wellcome Trust, and the Chan Zuckerberg Foundation. This project was also supported through a research collaboration between AstraZeneca UK Limited and the Medical Research Council (BSF30).
Further references
Identification of a core transcriptional program driving the human renal mesenchymal-to-epithelial transition. Ng-Blichfeldt, J-P., Stewart, BJ., Clatworthy, MR., Williams, JM., Röper, K. Developmental Cell
Katja’s page
AstraZeneca
Blue Sky Collaboration
Previous Insight on Research articles
Early transcriptional patterning of a forming tissue is essential to morphogenesis of tubular organ
Discovery of a key piece of the puzzle of tubular organ formation