

At the start of organ development during embryogenesis most animals consist of a simple polarised epithelial sheet of cells that transforms into complex 3D shapes over time. Correct organ shape is critical for proper organ function. But what determines organ shape? It is the shapes and arrangement of cells within an organ that ultimately determines its shape. And cell shape itself is determined by the cytoskeleton, in particular by the actomyosin cortex and microtubule filaments. My lab’s core interest is how cell shape is determined by the cytoskeleton and how neighbouring cells are coordinated with one another to determine organ shape.
Many of the important organ systems in mammals and in invertebrate model systems retain their epithelial nature, and are often tubular in structure. This is true for the intestinal tract, kidney, liver, lung, and vasculature. With defects in tube formation and failure in tube homeostasis leading to severe disease phenotypes including Spina Bifida or Polycystic Kidney Disease, it is clear that understanding the mechanisms that drive faithful organ morphogenesis is key to a better understanding, and eventually treatment, of such defects.
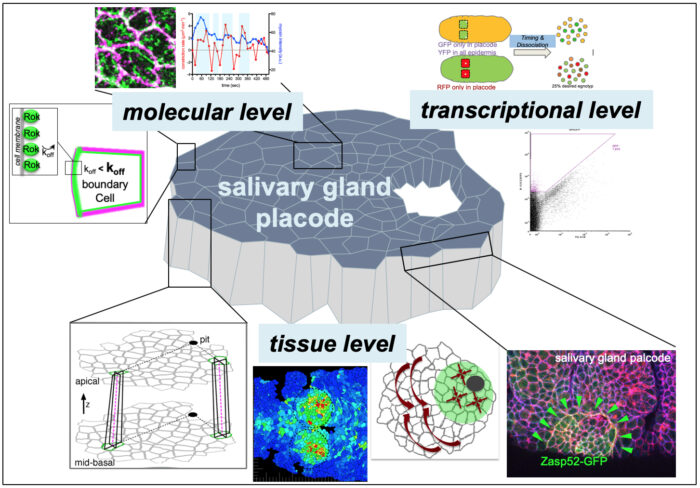
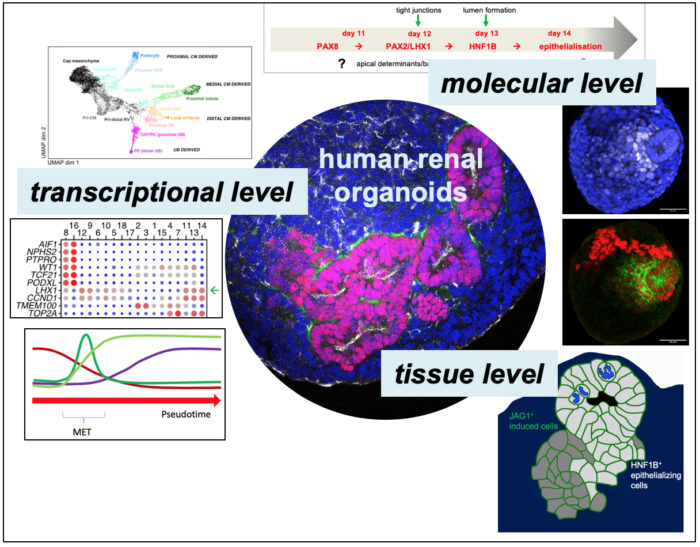
Tubular organs can form through a number of mechanisms, including folding and wrapping of, as well as budding from, epithelial sheets, but also through de novo formation of epithelial polarity. My lab’s research focusses on a two models of tube morphogenesis. One is the formation of the salivary glands in the Drosophila embryo through a budding process. This is a simple process of tube formation that is highly amenable to live imaging and genetic perturbation. Secondly, we use human renal organoids differentiated from stem cells to understand de novo generation of epithelial polarity and early nephron morphogenesis.
Using both systems, we address fundamental questions at different scales that will also inform our understanding of more complex processes of tissue morphogenesis.
Selected Papers
- Ashour, D.J., Durney, C.H., Planelles-Herrero, V.J., Stevens, T.J., Feng,J.J. and Röper, K. (2023)
Zasp52 strengthens whole embryo tissue integrity through supracellular actomyosin networks.
Development 7: dev201238. https://doi.org/10.1242/dev.201238 - Sánchez-Corrales, Y. E., Blanchard, G. B., & Röper, K. (2021)
Correct regionalisation of a tissue primordium is essential for coordinated morphogenesis.
eLife 10: e72369 https://doi.org/10.7554/eLife.72369 - Gillard, G., Girdler, G. & Röper, K. (2021)
A release-and-capture mechanism generates an essential non-centrosomal microtubule array during tube budding.
Nat Commun 12: 4096. https://doi.org/10.1038/s41467-021-24332-0 - Sidor, C., Stevens, T., Jin, L., Boulanger, J., Röper, K. (2020)
Rho kinase planar polarisation at tissue boundaries depends on phospho-regulation of membrane residence time.
Developmental Cell 52(3): 364-378. https://doi.org/10.1016/j.devcel.2019.12.003 - Sánchez-Corrales, Y. E., Blanchard, G. B., & Röper, K. (2018)
Radially-patterned cell behaviours during tube budding from an epithelium.
eLife 7: e35717. http://doi.org/10.7554/eLife.35717
Group Members
- Gabriella Symons
- Robert Turnbull