Cryo-EM is key to determining the structures of molecules responsible for life – a new specimen grid technology makes cryo-EM even better, faster, and cheaper
Electron cryo-microscopy (cryo-EM) has become an important imaging technique for determining the precise arrangements of atoms within protein structures, but specimen movement is still a problem that reduces data quality. Chris Russo’s group, in the LMB’s Structural Studies Division, has now presented a physical theory for the causes of this movement, and created a new specimen support that eliminates it.
When an electron beam is fired through a protein sample during imaging, the molecules immediately start moving around, blurring the images produced. By studying this movement, Chris’s group were able to understand what causes it and work out how to prevent it. Previously, Chris, in collaboration with Lori Passmore, had worked out one major reason the specimens moved – the material they were made of crinkled up when cooled to the extreme temperatures used in the microscope. By instead making the grid entirely from gold, they were able to reduce specimen movement by a factor of 50.
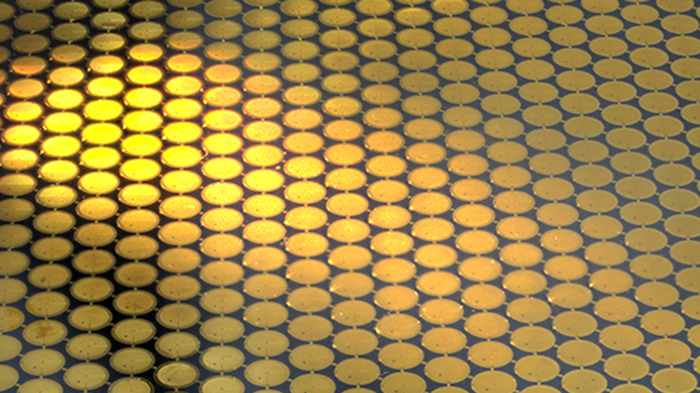
But all the movement was still not gone. Together with Katerina Naydenova, a PhD student in Chris’ group, they have now worked out a full physical theory of the causes of movement of cryo-EM specimens during imaging. With help from visiting post doc Peipei Jia from the University of Adelaide, an expert in nanofabrication, they have used this theory to create an improved all-gold grid that completely eliminates the movement of the ice surrounding the cryo-EM specimen.
Compared to the previous generation of all-gold grids, the new ones have 10 times smaller holes, which hold the protein molecules suspended in thin layers of transparent, glass-like ice. The diameter of one such hole is even smaller than the wavelength of visible light. The tiny holes grip the ice layer like a vice and prevent it moving even the tiniest amount – on average less than 1 Ångström or the diameter of a single hydrogen atom.
As well as directly improving data quality, movement-free imaging allowed Chris and Katerina to observe how the electron radiation damages the sample. They developed an algorithm that will enable researchers to work out what the protein structure was before the damage was done. “It’s like watching an explosion in reverse: we can ‘rewind the movie’ and see what the molecules looked like before the microscope was even turned on!” says Chris. This was previously impossible in cryo-EM, because the progression of damage could not be disentangled from erratic movement of the molecules and ice layer.
In addition to these improvements, the new grid will enable higher throughput of data collection. This is because the new hexagonal arrangement of holes on these grids increases the usable holes by a factor of 10 relative to standard cryo-EM grids. With these new grids, and the latest generation of electron microscopes and detectors, researchers will soon be able to routinely solve multiple protein structures in a day.
The core idea of structural biology is that we can better understand how proteins perform their functions by studying their precise three-dimensional structures. More detailed structures will allow design of more specific drugs that can regulate the function of target proteins. By improving the capacity of cryo-EM for high-resolution protein structure determination, these new grids could have a great impact on structure-based drug design for a wide range of diseases.
These latest cryo-EM technology developments were supported by the LMB mechanical and electronics workshops, electron microscopy facility and scientific computing, as well as the Cambridge Pharmaceutical Cryo-EM Consortium and Department of Materials Science and Metallurgy, University of Cambridge.
The work was funded by UKRI MRC; a Vice-Chancellor’s Award (Cambridge Commonwealth, European and International Trust); a Sir John Bradfield Scholarship from Trinity College, Cambridge; and the Australian Nanotechnology Network.
Further references
Cryo-EM with sub–1 Å specimen movement. Naydenova, K., Jia, P., Russo, CJ. Science 370(6513): 223-226.
Chris’ group page
Science Perspective: Better, faster, and even cheap