New study uncovers a unique molecular “clamp and pin” mechanism that activates the D2l complex, which then recruits enzymes that cut out damaged DNA
Our DNA is the instruction manual to all the tasks our cells need to perform: from growth to replication, and even to their demise. Understandably, the DNA helix must be very stable, and any damage to it can cause cells to act abnormally, such as grow uncontrollably (in the case of cancer). DNA is also often under attack either from external factors such as alcohol, UV rays or internal cellular by-products.
Although these factors consistently bombard our DNA, the damage is often undone as our cells also have the ability to repair DNA through intricate mechanisms. However, DNA repair does not always work. For example, patients suffering from Fanconi Anaemia (FA) have mutations that prevent the efficient repair of a certain type of DNA damage, known as inter-strand crosslinks or ICLs (What is a DNA crosslink?). This leads to the loss of blood production, developmental defects and predisposition to cancer. But this disease has also allowed scientists to understand how chemotherapy works.
To understand what causes FA, scientists need to decipher each and every move the proteins and the complexes make while repairing DNA through the FA pathway. This study, led by Pablo Alcón and Shabih Shakeel in Lori Passmore’s group, in collaboration with KJ Patel in the LMB’s PNAC Division and Juri Rappsilber’s group at Technische Universitat Berlin, reveals a sophisticated mechanism that answers a long-standing question in the field of DNA repair.
The activation of the protein complex known as D2l is crucial to this pathway because this triggers the excision of damaged DNA, and its subsequent repair. The activation happens when the FA core complex (a large composite of proteins that activates DNA repair) adds one ubiquitin molecule to D2I, but the exact mechanism of this process has remained unknown.
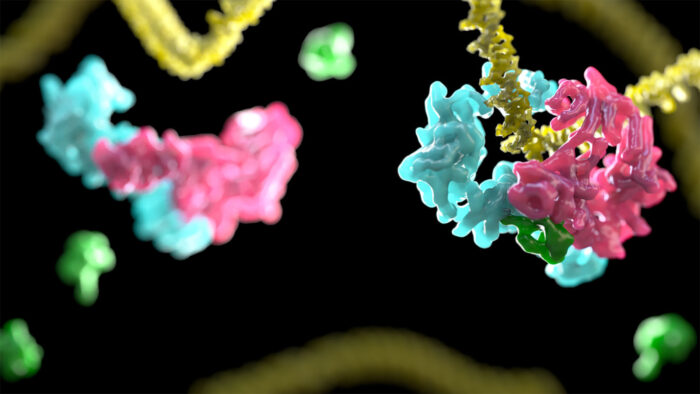
Movie showing transition from open to closed DNA clamp. (D2I in pink and blue, DNA in yellow, and ubiquitin in green)
Tiny movements, big impact
This study shows that the binding to DNA causes D2I, which normally looks like an open clamp, to change its shape. The clamp closes, fastening the protein complex tightly onto the DNA strand. D2I (made of proteins FANCD2 and FANCI) resembles two saxophones lying head-to-toe. These two “saxophones” swivel, creating a channel that embraces the DNA. Then ubiquitin, added by the core complex, is wedged between FANCD2 and FANCI, locking this closed conformation. The scientists propose that this “locked D2I” is the platform that marks the DNA as damaged and recruits enzymes which cut open the DNA. These parts can then be replaced by a healthy copy.
Uncovering such mechanistic details at the molecular level is fundamental to understanding how the entire FA pathway functions. This kind of basic science is of paramount importance at the LMB. “This is a clear example of the relevance of elucidating cellular mechanisms and how dissecting them impacts our understanding of a range of diseases and treatments.” says Pablo Alcón.
From trenches to lab benches: The history of ICL
Mustard gas was a deadly chemical weapon first used in World War I against the allies. It killed, maimed and inflicted terror on battlefields near the end of the Great War. By the time of the Second World War, the Allied Forces were anxious about a repeat of the first war. Scientists got busy looking for antidotes to the toxin. This is when two scientists from Yale University, Louis Goodman and Alfred Gilman, discovered that the gas particularly affected white blood cells (which when mutated can develop into cancers), and hypothesised that this agent could be used to treat leukemia. Later on, it was discovered that these and similar anti-cancer drugs worked by generating DNA ICLs. Since then, agents that induce ICLs are commonly used in chemotherapy. Therefore, understanding ICL repair is important not only to understand the molecular mechanism of Fanconi anaemia, but also DNA repair after chemotherapy.
A long-standing research question
This is a good example of how science works step by step, and researchers build upon each other’s work, passing the baton forward. LMB scientists including Lori Passmore, KJ Patel, and other scientific groups, have worked to painstakingly put together the screenplay of this intricate biochemical mechanism that allows our cells to repair its damaged DNA. It has been a long scientific quest since 2011 when an LMB-Wellcome Sanger Institute-CRUK Cambridge Institute team developed the first FA model. This study sets the stage for future work to ultimately obtain a complete view of DNA inter-strand crosslink repair.
The work was funded by the MRC, German Research Foundation, the Wellcome Trust and an EMBO long-term fellowship.
Further references
FANCD2–FANCI is a clamp stabilized on DNA by monoubiquitination of FANCD2 during DNA repair. Alcón, P., Shakeel, S., Chen, ZA., Rappsilber, J., Patel, KJ. and Passmore, L. Nature Structural and Molecular Biology DOI: 10.1038/s41594-020-0380-1
Lori’s group page
Juri Rappsilber’s group page
Previous Insights on Research
Breakthrough in Fanconi anaemia research, Feb 10, 2011
Insights into how the Fanconi Anaemia core complex activates DNA repair, June 6, 2014
A fundamental protection mechanism against formalin in mammals is revealed, Sept 25, 2015
Decade-long collaboration results in the first structure of the Fanconi anaemia core complex, Oct 31, 2019