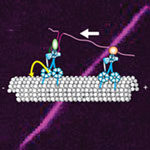
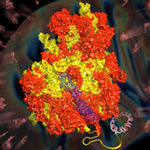
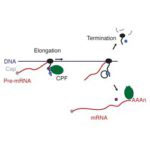
Lori Passmore and Chris Russo from the LMB’s Structural Studies Division have discovered how to modify the new material graphene using low-energy hydrogen plasmas, to allow it to bind proteins. This discovery makes it suitable for use in electron microscopy to solve protein structures.
Graphene, which was only discovered in 2004, is a two-dimensional carbon sheet that is one of the strongest and thinnest materials known.