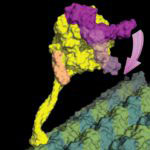
When a bacterial cell divides, the cell membrane and cell envelope have to pinch together in the middle of the cell to separate it into two daughter cells. A ring of proteins called the divisome constricts, cleaving the cell in two. The protein FtsZ is a crucial component of this ring and many FtsZ subunits join together in a chain forming long filaments. These FtsZ filaments are anchored to the membrane by another protein, FtsA, so that the membrane also constricts when the FtsZ ring closes.