MORPHEUS III protein crystallization screen (2017)
MORPHEUS III is a 96-condition 3D grid screen for protein crystallisation invented at the MRC Laboratory of Molecular Biology by Dr Fabrice Gorrec. The screen has been formulated to solve novel structures of proteins and macromolecular complexes. For this, the main ideas behind the formulation of MORPHEUS I (2009) and MORPHEUS II (2015) were followed, with essentially:
- to increase the yield of quality diffraction crystals by integrating mixes of additives that can act as protein stabilisers, crystal cross-linkers, etc. (a feature initially investigated by McPherson & Cudney in 2006 for their ‘Silver Bullets’ screen).
- systematic approaches to select the reagents and formulate the screen, like the integration of ligands that are found in crystal structures of the Protein Data Bank (PDB).
- considering pragmatic aspects that facilitate the steps towards structure solution. For example, cryoprotectants are integrated for straightforward flash-freezing of crystals.
- testing formulation prototypes on a broad range of samples purified at the LMB in order to avoid biases towards a specific subset/class of targets.
The novelty in MORPHEUS III is based on the integration of small drug-like compounds as additives (some of which were found in other databases than the PDB). The final formulation of the new 96-condition crystallisation screen integrates 44 compounds overall selected for their relative stability and solubility. These compounds were divided in 8 mixes of additives in order to formulate the typical MORPHEUS 3D grid screen where each mix of additive is combined with 4 cryoprotected precipitant mixes and 3 buffer systems to form the 3D grid (see Figure 1).
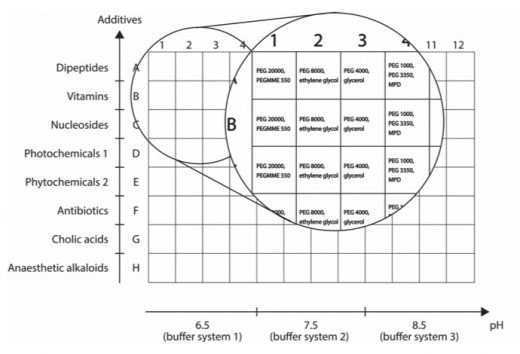
The set of single compounds can be found in an additive screen called HIPPOCRATES. LifeArc commercialised MORPHEUS® and HIPPOCRATES screens under an exclusive licence to Molecular Dimensions.
Gorrec, F. (2009)
The Morpheus protein crystallisation screen.
J Appl Cryst 7, 1035-1042.
Gorrec, F. (2015)
The Morpheus II protein crystallisation screen.
Acta Cryst F 71, 831-837.
Gorrec, F. and Zinzalla, G. (2018)
The MORPHEUS III protein crystallization screen: at the frontier of drug discovery.
IUCr Newsletter 26.