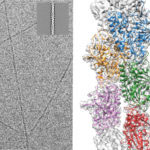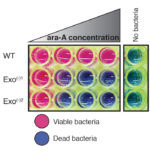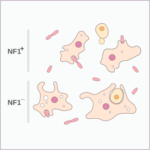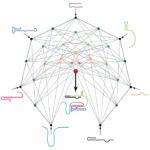
There is compelling evidence that in the distant past, our single-celled ancestors used RNA, a chemical cousin of DNA, for both genetic information storage and metabolism. This primordial “RNA world” would have needed an RNA enzyme able to replicate itself and other primordial “RNA genes”.