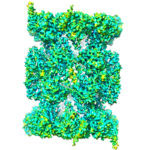
The proteasome is a protein recycling complex that plays a critical role in the smooth running of our cells. It is present in all multicellular organisms and facilitates cell renewal and the controlled death of damaged cells.
MRC Laboratory of Molecular Biology
One of the world's leading research institutes, our scientists are working to advance understanding of biological processes at the molecular level - providing the knowledge needed to solve key problems in human health.
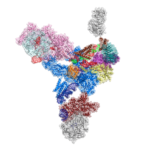
Genes in eukaryotic organisms are frequently interrupted by non-coding segments called introns. Before the protein can be produced, the entire length of each gene including the introns, is transcribed to produce precursor messenger RNA (pre-mRNA). An immense and dynamic molecular machine known as the spliceosome then removes the introns and splices the coding segments together to form mature mRNA, which can then be translated into proteins.
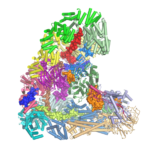
The anaphase-promoting complex (APC/C) is an unusually large multi-subunit complex that has a key role in cell division, controlling chromosome segregation and the cell cycle. Using electron cryo-microscopy (cryo-EM), David Barford and his group in the LMB’s Structural Studies Division have solved the first atomic structure of the APC/C, revealing its molecular architecture in unprecedented detail.
When cells divide they go through a series of phases known as the cell cycle.
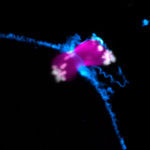
Miscarriages and genetic disorders, such as Down’s syndrome, are often caused by errors when an egg develops from its progenitor cell, the oocyte. Most defects occur during meiosis, a specialised form of cell division that leads to the formation of an egg. To ensure the egg cell has exactly the right number of chromosomes, an oocyte has to separate away and remove half of its chromosomes.
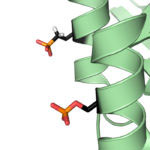
Protein phosphorylation is a key post-translational modification that is used to regulate many essential cell processes. A phosphate group is added to a specific site in a protein to switch that protein on or off, depending on its role. It has been challenging to understand the function of protein phosphorylation because it is difficult to make phosphorylated proteins. However, a new method developed by Jason Chin’s group in the LMB’s PNAC Division could revolutionise this research.
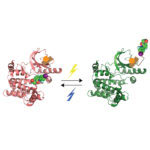
Small molecules are often used to regulate the functions of proteins and provide many drugs and tools for understanding biological pathways. However, selectively controlling a specific target protein within living cells is a major challenge. Jason Chin’s group from the LMB’s PNAC Division have created a new technique called BOLT that can selectively regulate specific proteins inside cells that could not previously be targeted.