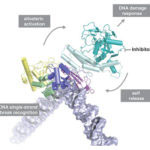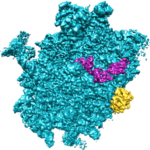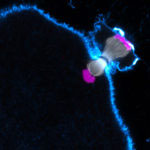
Human eggs are frequently aneuploid, meaning they have the wrong number of chromosomes, and this is a major cause of pregnancy loss and Down syndrome. Aneuploidy in human eggs increases with advanced maternal age, which may explain why it is more difficult for women to get pregnant as they get older, and why miscarriages and Down syndrome are more likely in women of advanced age. However, the causes of this maternal age effect in humans have until recently been largely unclear.