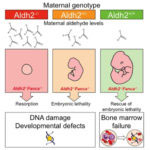
Whilst a mother’s metabolism provides essential nutrients to enable embryonic development, both mother and embryo can also produce reactive metabolites that can damage DNA. Research undertaken by Nina Oberbeck in KJ Patel’s group, in the LMB’s PNAC Division, has uncovered how the embryo is protected from these genotoxins.
Birth defects are common and are a substantial burden to human health, but their causes are complex and often due to many factors.