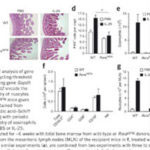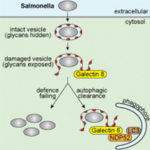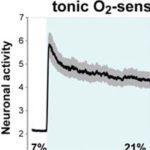
A group of researchers, led by Mario de Bono’s group in the LMB’s Cell Biology division, have extended our understanding of how animals respond to long-term dangers at a molecular level – which may help in explaining how this process fails in a range of human diseases and medical conditions.
Sensory neurons send the brain a barrage of environmental information. Most of these neurons react briefly to short-term stimuli and ignore the stimulus if it persists.