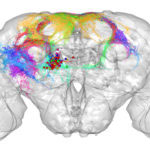
Researchers in Greg Jefferis’s group in the LMB’s Neurobiology Division have developed a new online tool to analyse images of neurons. This tool, known as NBLAST, is free and available to all. NBLAST enables researchers to measure the similarity between neurons and organise them into neuron families, akin to tools such as BLAST that allow protein sequences to be compared.
Neuroscience is seeing a period of major growth in the structural characterisation of neurons.