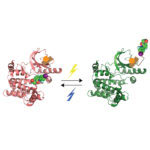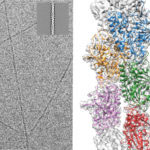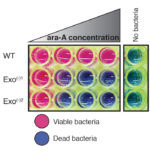
Protein phosphorylation is a key post-translational modification that is used to regulate many essential cell processes. A phosphate group is added to a specific site in a protein to switch that protein on or off, depending on its role. It has been challenging to understand the function of protein phosphorylation because it is difficult to make phosphorylated proteins. However, a new method developed by Jason Chin’s group in the LMB’s PNAC Division could revolutionise this research.