

Integral membrane proteins are fundamental to a cell’s survival, allowing the import of nutrients, the export of toxins and intercellular communication via receptors. We aim to understand the processes of solute translocation and receptor signalling processes by determining the structures of important and interesting membrane proteins by x-ray crystallography. The current particular focus of the lab are G protein-coupled receptors (GPCRs).
There are over 350 non-odorant GPCRs encoded by the human genome that are involved in hormone binding and transducing this signal to the cytoplasm, resulting in the activation of G protein pathways and eventually eliciting a cellular response. The key role of GPCRs in signal transduction pathways makes them ideal as drug targets with many classes of small molecule drugs, such as beta blockers or anti-asthma treatments, either inhibiting or activating these receptors. There are many potential problems in determining the structure of membrane proteins, but we have developed a strategy based on rational mutagenesis to improve the thermostability of GPCRs, which has made crystallisation and structure determination far more tractable. We have now determined multiple structures of the ?1adrenergic receptor and adenosine A2A receptor bound either to full agonists, partial agonists, inverse agonists or biased agonists. This has led to a molecular understanding of ligand efficacy at these receptors.
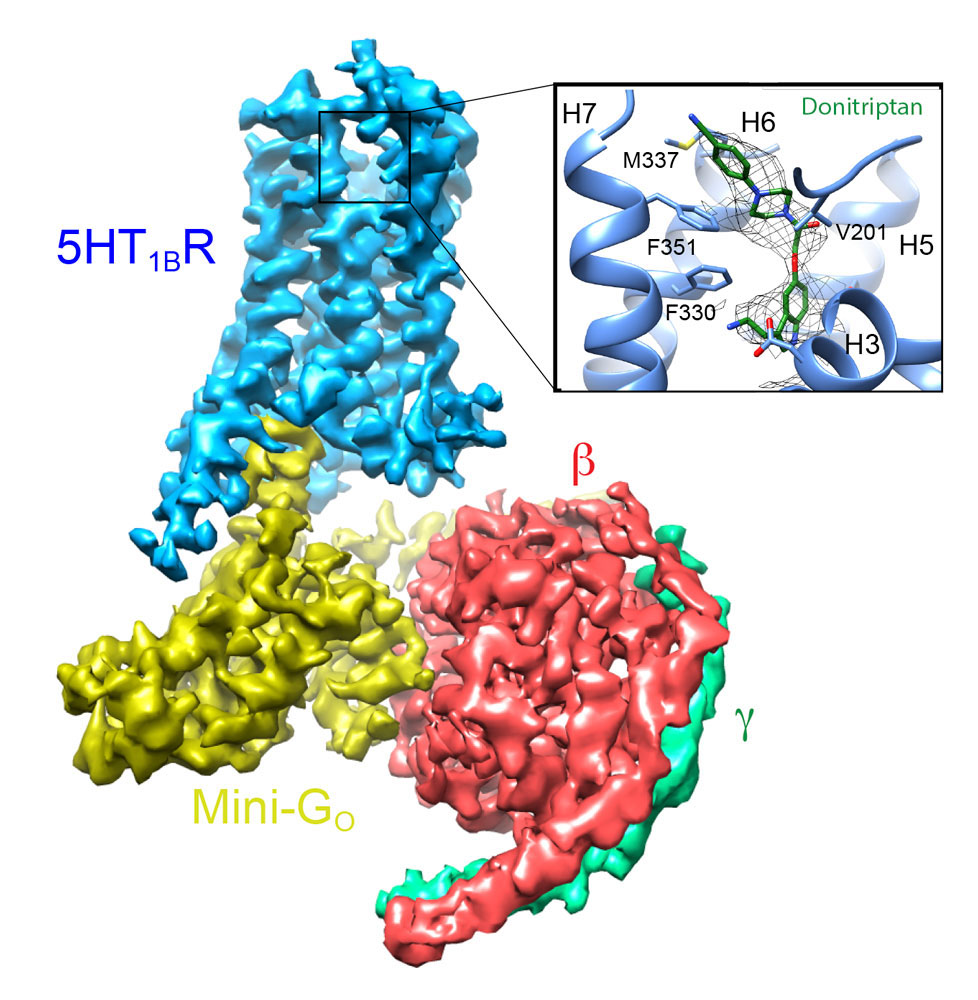
Determining the structures of GPCRs in the fully active state coupled to G proteins is also very challenging. We have approached this problem by developing an engineered β-subunit of the heterotrimeric G protein, which we call a mini-G protein. This recapitulates all the pharmacology of a native G protein coupling to a GPCR, but is a fraction of the size, thus allowing the formation of well-diffracting crystals. This has led to the structure determination of the adenosine A2A receptor in the fully active state and, more recently, rhodopsin. In addition, we have used these engineered G proteins to facilitate the structure determination of GPCR complexes by cryo-EM. We have recently determined the first structure of a Go coupled receptor, the serotonin 5HTIB receptor, which is facilitating our understanding of the specificity of G protein coupling.
Structure of the adenosine A2A receptor
coupled to mini-Gs.
Selected Papers
- Velazhahan, V., Ma, N., Vaidehi, N. & Tate, C.G. (2022)
Activation mechanism of the class D fungal GPCR dimer Ste2.
Nature 603: 743-748. - Velazhahan, V., Ma, N., Pándy-Szekeres, G., Kooistra, A.J., Lee, Y., Gloriam, D.E., Vaidehi, N. & Tate, C.G. (2021)
Dimeric structure of the Class D GPCR Ste2 coupled to two G proteins.
Nature 589: 148-153. - Lee, Y., Warne, T., Nehmé, R., Pandey, S., Dwivedi-Agnihotri, H., Edwards, P.C., García-Nafría, J., Leslie, A.G.W., Shukla, A.K. & Tate, C.G. (2020)
Molecular determinants of β-arrestin coupling to formoterol-bound β1-adrenoceptor.
Nature 583: 862-866. - Warne, T., Edwards, P.C. Doré, A.S., Leslie, A.G.W. & Tate, C.G. (2019)
Molecular basis for high-affinity agonist binding in GPCRs.
Science 364: 775-778. - García-Nafría, J., Nehmé, R., Edwards, P.C. & Tate, C.G. (2018)
Cryo-EM structure of the serotonin 5-HT1B receptor coupled to heterotrimeric Go.
Nature 558: 620-623. - Carpenter, B., Nehmé, R., Warne, T., Leslie, A.G.W. & Tate C.G. (2016)
Structure of the adenosine A2A receptor bound to an engineered G protein.
Nature 536: 104-107. - White, J.F., Noinaj, N., Shibata, Y., Love, J., Kloss, B., Xu, F., Gvozdenovic-Jeremic, J., Shah, P., Shiloach, J., Tate, C.G. and Grisshammer, R. (2012)
Structure of the agonist-bound neurotensin receptor.
Nature 490: 508-513. - Warne, T., Moukhametzianov, R., Baker, J.G., Nehme, R., Edwards, P.C., Henderson, R., Leslie, A.G.W., Schertler, G.F.X. and Tate, C.G. (2011)
The structural basis for agonist and partial agonist action on a β1-adrenergic receptor.
Nature 469: 241-244. - Lebon, G., Warne, T., Edwards, P.C., Bennett, K., Langmead, C.J., Leslie A.G.W. and Tate C.G. (2011)
Agonist-bound structures of the adenosine A2A reveals common features of GPCR activation.
Nature 474: 521-525.
Group Members
- Qingchao Chen
- David Favara
- Adelaine Leung
- Jade Li
- Yue Ren
- Malcolm Weir