

Despite extracellular immune surveillance, viral and bacterial pathogens are still able to infect cells. This means each cell must have its own mechanisms for directly restricting pathogen replication. It is this process of intracellular immunity that my lab is interested in. We take a hypothesis-driven approach and investigate infection from both the host and pathogen perspective. The evolutionary arms race between the two continues to provide some of the most unexpected and fascinating discoveries in biology and these insights form the basis of future medicines and therapies.
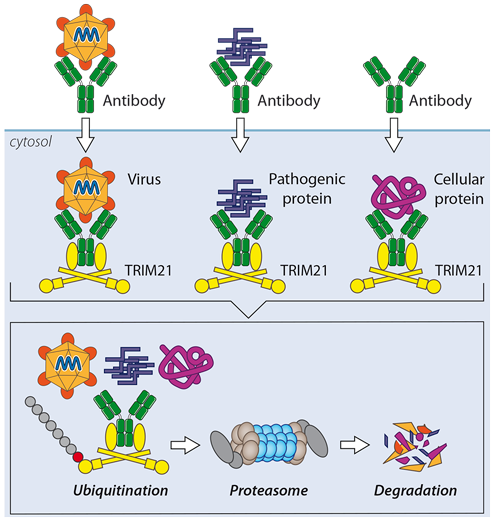
One of our key discoveries is the cytosolic antibody receptor TRIM21. TRIM21 is expressed in every cell and combines innate and adaptive immune mechanisms to protect us from infection. TRIM21 mediates the rapid proteasomal degradation of viruses, pathogenic prions and even cellular proteins. This latter activity we have exploited in a technique called TrimAway that allows rapid and specific protein depletion in any cell.
We utilize a wide-range of techniques from biophysics to cellular and in vivo models of infection. In this way, we hope to understand how function is transmitted from molecules to cells.
Movie 1: Cells expressing TRIM21 and GFP are microinjected with anti-GFP IgG antibodies. TRIM21 rapidly detects the GFP-IgG complexes and forms cytoplasmic bodies. TRIM21 recruits the proteasome to degrade the complexes, specifically depleting GFP from the cell. Inhibiting the proteasome with MG132 prevents TrimAway.
Movie 2: Taking X-ray snapshots of the molecular structure of HIV-1 capsid hexamers and turning them into a movie helped us discover dynamic pores in the viral protein coat. These pores are controlled by the movement of a β-hairpin motif that opens and closes like the iris of an eye.
Selected Papers
- Renner, N., Kleinpeter, A., Mallery, D.L., Albecka, A., Faysal, K.M.R., Böcking, T., Saiardi, A., Freed, E.O., James, L.C. (2023)
HIV-1 is dependent on its immature lattice to recruit IP6 for mature capsid assembly
Nature Structural & Molecular Biology, doi: 10.1038/s41594-022-00887-4. - Mallery DL, Kleinpeter AB, Renner N, Faysal KMR, Novikova M, Kiss L, Wilson MSC, Ahsan B, Ke Z, Briggs JAG, Saiardi A, Böcking T, Freed EO, James LC. (2021)
A stable immature lattice packages IP6 for HIV capsid maturation.
Science Advances 7(11): eabe4716. doi: 10.1126/sciadv.abe4716. - Zeng J, Santos AF, Mukadam AS, Osswald M, Jacques DA, Dickson CF, McLaughlin SH, Johnson CM, Kiss L, Luptak J, Renner N, Vaysburd M, McEwan WA, Morais-de-Sá E, Clift D, James LC. (2021)
Target-induced clustering activates Trim-Away of pathogens and proteins.
Nat Struct Mol Biol. 28(3): 278-289. doi: 10.1038/s41594-021-00560-2. - Caddy SL, Vaysburd M, Papa G, Wing M, O’Connell K, Stoycheva D, Foss S, Andersen JT, Oxenius A, James LC. (2021)
Viral nucleoprotein antibodies activate TRIM21 and induce T cell immunity
EMBO J., doi.org/10.15252/embj.2020106228 - Clift D, McEwan WA, Labzin LI, Konieczny V, Mogessie B, James LC*, Schuh M*. (2017)
A Method for the Acute and Rapid Degradation of Endogenous Proteins.
Cell 171(7): pii: S0092-8674(17)31255-2. (*co-corresponding authors) - Jacques DA, McEwan WA, Hilditch L, Price AJ, Towers GJ, James LC. (2016)
HIV-1 uses dynamic capsid pores to import nucleotides and fuel encapsidated DNA synthesis.
Nature 536: 349-53. - Tam, J.C., Bidgood, S.R., McEwan, W.A. and James, L.C. (2014)
Intracellular sensing of complement C3 activates cell autonomous immunity
Science 345(6201): p. 1256070.
Group Members
- Anna Albecka-Moreau
- Dean Clift
- Aminu Jahun
- Janek Ole Klarhof
- Jakub Luptak
- Donna Mallery
- Lauren Miller
- Tyler Rhinesmith
- Beth Thompson
- Boglarka Anna Vamos
- Marina Vaysburd