

Cellular physiology relies on the accurate deployment of every gene product to the correct cellular compartment. Fully one third of the proteins encoded in eukaryotic genomes navigate the secretory pathway, entering this system via the endoplasmic reticulum (ER). Research in the Miller lab is broadly aimed at understanding basic mechanisms of secretory protein biogenesis, focusing on protein quality control within the ER. We use the budding yeast, Saccharomyces cerevisiae, as a model system, which affords facile biochemical, genetic, genomic and proteomic tools. By using such a tractable model system, we can rapidly discover new pathways and dissect mechanisms that may be directly relevant to a number of human diseases, most notably cystic fibrosis and similar diseases of protein misfolding.
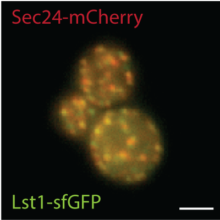
The molecular basis for vesicle formation from the ER is relatively well understood and relies on cytoplasmic coat proteins known as the COPII coat. Yet, despite a relatively deep understanding of the mechanisms that drive COPII vesicle formation and cargo capture, we know very little about how this process is regulated to prevent improper traffic of misfolded proteins. We study this problem from two angles: a “cargo-centric” approach examining the folding and trafficking of an individual protein, and a systems-level approach to characterize quality control more broadly. We use high throughput yeast genetics to identify new components that act in various aspects of protein quality control, and biochemical approaches to dissect mechanism. One long-term goal is to understand how vesicle abundance and architecture can adapt to changing physiological needs with respect to cargo load.
Selected Papers
- Stancheva, V.G., Li, X-H., Hutchings, J., Gomez-Navarro, N., Santhanam, B., Madan Babu, M., Zanetti, G., Miller, E.A. (2020)
Combinatorial multivalent interactions drive cooperative assembly of the COPII coat.
J. Cell. Biol . 219(11): e202007135. doi: 10.1083/jcb.202007135. - Gomez-Navarro, N., Melero, A., Li, X.H., Boulanger, J., Kukulski, W. and Miller, E.A. (2020)
Cargo crowding contributes to sorting stringency in COPII vesicles.
J. Cell. Biol. 219(7): e201806038. - Lakshminarayan, R., Phillips, B.P., Binnian, I.L., Gomez-Navarro, N., Erscudero-Urquijo, N., Warren, A.J. and Miller, E.A. (2020)
Pre-emptive quality control of a misfolded membrane protein by risbosome-driven effects.
Current Biol. 30(5): 854-864. - Hutchings, J., Stancheva, V., Miller, E.A. and Zanetti, G. (2018)
Subtomogram averaging of COPII assemblies reveals how coat organization dictates membrane shape.
Nat. Commun. 9(1): 4154. - Pagant, S., Wu, A., Edwards, S. and Miller, E.A. (2015)
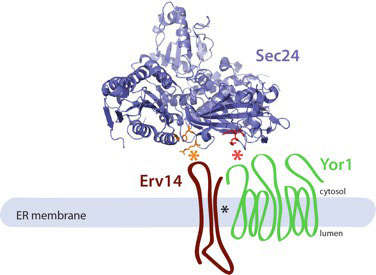
Sec24 is a coincidence detector that binds both cargo proteins and the cargo receptor, Erv14, to drive ER export.
Current Biology 25: 403-412. - Copic, A., Latham, C.F., Horlbeck, M., D’Arcangelo, J.G. and Miller, E.A. (2012)
ER cargo properties specify a requirement for COPII coat rigidity mediated by Sec13p.
Science 335: 1359-1362.
Group Members
- Ying Bai
- Ivan Domenech Mercade
- Julija Maldutyte
- Sarah Triclin