

The compartments of the secretory and endocytic pathways are connected by membrane-bound carriers that bud from, and then fuse with, specific organelles. The accuracy of this traffic depends on the organelles having an ‘identity’ by which the trafficking machinery can recognise them. This identity is defined by organelle-specific G proteins of the Arf and Rab families and by phosphoinositide lipids.
We are interested in how active G proteins are generated in a spatially restricted manner, and what cytosolic proteins or ‘effectors’ then recognise them.
Our work is focussed on the Golgi apparatus, an organelle that plays a central role in the sorting and modification of proteins in the secretory pathway. The Golgi contains a number of Arf and Rab proteins, and we apply biochemical and genetic methods to identify their interacting partners both in mammalian tissue culture cells and in Drosophila.
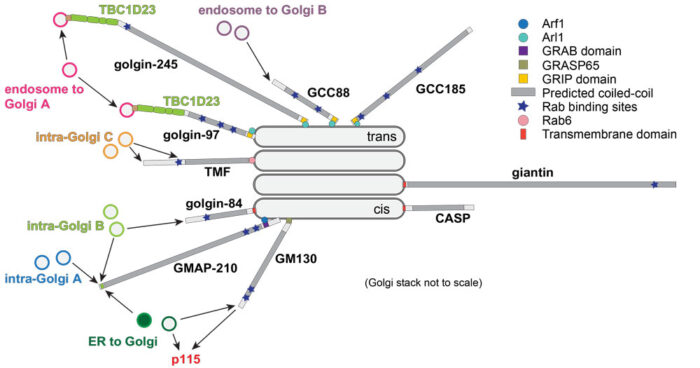
One major class of effector that we are investigating are the long coiled-coil proteins that are believed to act in the tethering of carriers prior to fusion.
An understanding of how organelle identity is established and recognised is only starting to emerge but promises to reveal much about the underlying logic of the organisation of eukaryotic cells.
Selected Papers
- Cattin-Ortolá J, Kaufman JGG, Gillingham AK, Wagstaff JL, Peak-Chew SY, Stevens TJ, Boulanger J, Owen DJ, Munro S. (2024)
Cargo selective vesicle tethering: The structural basis for binding of specific cargo proteins by the Golgi tether component TBC1D23
Sci Adv. 10(13): eadl0608 - Rocha, J.J, Jayaram, S.A., Stevens, T.J., Muschalik,N , Shah, R.D. Emran, S., Robles, C., Freeman, M., Munro, S. (2023)
Functional unknomics: Systematic screening of conserved genes of unknown function
PLoS Biol 21(8): e3002222 - Sung Yun Park, Nadine Muschalik, Jessica Chadwick, Sean Munro (2022)
In vivo characterization of Drosophila golgins reveals redundancy and plasticity of vesicle capture at the Golgi apparatus
Curr Biol 32(21): 4549-4564 - Lawrence G Welch, Sew-Yeu Peak-Chew, Farida Begum, Tim J Stevens, Sean Munro (2021)
GOLPH3 and GOLPH3L are broad-spectrum COPI adaptors for sorting into intra-Golgi transport vesicles
J Cell Biol . 220(10): e202106115
Group Members
- Katherine Brown
- Jessica Chadwick
- Antonio Galindo
- Alison Gillingham
- Shubham Kumar
- Nadine Muschalik
- David Owen
- Emma Stone
- Alexander Van Vliet
- Nikita Zubkov