

TB remains a major cause of death despite a live attenuated vaccine (BCG) for a century and effective antibiotics for 60 years. TB’s persistence over millennia in the face of these major medical advances underscores the ability of Mycobacterium tuberculosis to evade and exploit host defenses and antibiotics. Pathogenic mycobacteria infect macrophages and survive in these primary immune defense cells by subverting their endocytic trafficking and microbicidal mechanisms. The bacteria also alter macrophage differentiation, migration, and aggregation to induce the formation of granulomas, enigmatic, complex, organized immune structures that can paradoxically promote bacterial growth. Ultimately, mycobacteria can cause granuloma breakdown by inducing programmed macrophage death, a step that further increases bacterial growth and promotes transmission to new hosts.
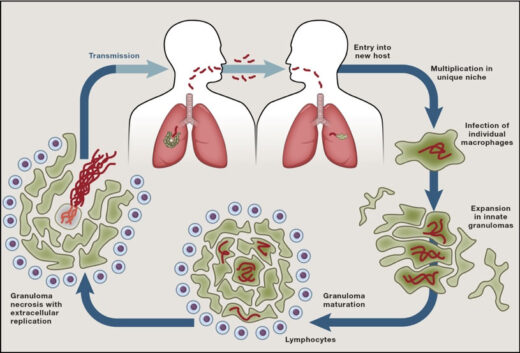
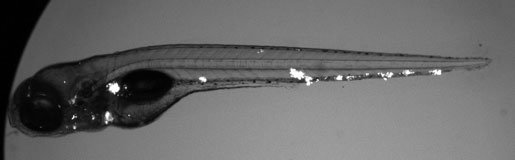
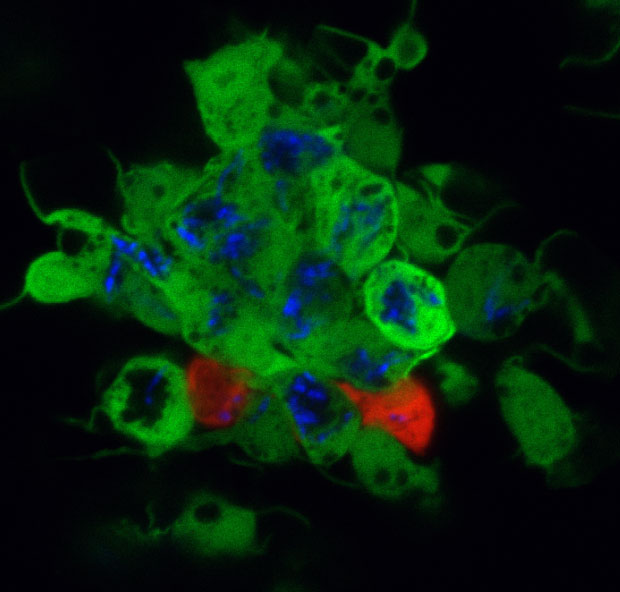
We use the zebrafish larva as a surrogate model organism to study TB, exploiting its optical transparency and genetic and pharmacological tractability to monitor infection in real-time. Through genetic screens, we identify host susceptibility and resistance factors and bacterial virulence determinants that alter specific infection steps. Our research is shedding light on TB pathogenesis as well as fundamental macrophage biology – organellar cross-talk, migration, adhesion and death. Our findings have been borne out in humans and are informing new treatment strategies. We have also used the zebrafish to unravel mysteries in other granulomatous diseases like leprosy and schistosomiasis.
Selected Papers
- Francisco J. Roca, Laura J. Whitworth, Hiran A. Prag, Michael P. Murphy, Lalita Ramakrishnan (2022)
Tumor necrosis factor induces pathogenic mitochondrial ROS in tuberculosis through reverse electron transport
Science 376: 1431 - F.J Roca, L.J. Whitworth, S. Redmond, A.A. Jones, L. Ramakrishnan (2019)
TNF induces pathogenic programmed macrophage necrosis in tuberculosis through a mitochondrial-lysosomal-endoplasmic reticulum circuit.
Cell 178: 1344-1361. - C.A. Madigan, C.J. Cambier, K.M. Kelly-Scumpia, P.O. Scumpia, T.Y. Cheng, J. Zailaa, B.R. Bloom, D.B. Moody, S.T. Smale, A. Sagasti, R.L. Modlin, L. Ramakrishnan. (2017)
A macrophage response to Mycobacterium leprae phenolic glycolipid initiates nerve damage in leprosy.
Cell 170: 973-985. - R.D. Berg, S. Levitte, M.P. O'Sullivan, S.M. O'Leary, C.J. Cambier, J. Cameron, K.K. Takaki, C.B. Moens, D.M. Tobin, J. Keane, L. Ramakrishnan (2016)
Lysosomal disorders drive susceptibility to tuberculosis by compromising macrophage migration.
Cell 165: 139-52. - C.J. Cambier, K.K. Takaki, R.P. Larson, R.E. Hernandez, D.M. Tobin, K.B. Urdahl, C.L. Cosma, L. Ramakrishnan (2014)
Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids.
Nature 505: 218-22. - D.M. Tobin, F.J. Roca, S.F. Oh, R. McFarland, T.W. Vickery, J.P. Ray, D.C. Ko, Y. Zou, N.D. Bang, T.T. Chau, J.C. Vary, T.R. Hawn, S.J. Dunstan, J. J. Farrar, G.E. Thwaites, M.C. King, C.N. Serhan, L. Ramakrishnan (2012)
Host genotype directed therapies can optimize the inflammatory response to mycobacterial infections.
Cell 148: 434-46. - K.N. Adams, K.K Takaki, L.E. Connolly, H. Wiedenhoft, K. Winglee, O. Humbert, P.E. Edelstein, C.L. Cosma, L. Ramakrishnan (2011)
Drug tolerance in replicating mycobacteria induced by a macrophage-induced efflux mechanism.
Cell 145: 39-53. - J.M. Davis and L. Ramakrishnan (2009)
The role of the granuloma in the expansion and dissemination of early tuberculous infection.
Cell 136: 39-53.