

Pluripotent stem cells have the unique capacity to generate all the cell types of the organism. In the embryo, they undergo concomitant changes in shape and identity to set the foundation of the body plan. What are the molecular mechanisms that coordinate shape and identity? And once a well-defined tissue architecture is acquired, how does it modulate pluripotent stem cell identity, differentiation and fate? To address these questions our group focuses on epithelial tissue determinants and their contribution to stem cell fate. Our final aim is to understand the molecular basis of human embryogenesis, and how the exquisite coordination between fate and shape is affected when development fails.
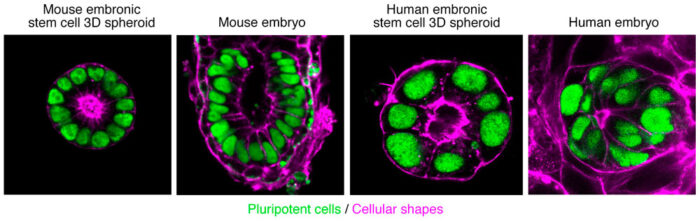
Pluripotent stem cells are not unique to embryos, as they can also be induced in adult tissues through transcription factor-mediated reprogramming. This represents a promising avenue for tissue regeneration and repair. In a clear parallel with the embryo, how adult epithelial tissue architecture modulates cellular identity, plasticity and fate remains unexplored. We seek to identify conserved molecular pathways that coordinate shape and fate during pluripotency exit and pluripotency acquisition.
To achieve our aim we deconstruct molecular mechanisms while reconstructing embryonic and adult tissues from stem cells. We anticipate these studies will uncover the molecular rules that direct morphogenesis and regeneration.
Selected Papers
- Makhlouf, A., Wang, A., Sato, N., Rosa, V.S., Shahbazi, M.N. (2024)
Integrin signaling in pluripotent cells acts as a gatekeeper of mouse germline entry
Sci Adv 10(36): eadk2252 - Sato N., Rosa VS., Makhlouf A., Kretzmer H., Kumar AS., Grosswendt S., Mattei A., Courbot O., Wolf S., Boulanger J., Langevin F., Wiacek M., Karpinski D., Elosegui-Artola A., Meissner A., Zernicka-Goetz M., Shahbazi MN (2024)
Basal delamination during mouse gastrulation primes pluripotent cells for differentiation.
Dev Cell 24: S1534-5807 - Shahbaz MN and Pasque V. (2024)
Early human development and stem cell-based human embryo models
Cell Stem Cell 31(10): 1398-1418 - Shahbazi MN (2020)
Mechanisms of human embryo development: from cell fate to tissue shape and back.
Developmen 147: dev190629. - Shahbazi MN., Scialdone A., Skorupska N., Weberling A., Recher G., Zhu M., Jedrusik A., Devito LG., Noli L., Macaulay IC., Buecker C., Khalaf Y., Ilic D., Voet T., Maironi JC., Zernicka-Goetz M. (2017)
Pluripotent state transitions coordinate morphogenesis in mouse and human embryos.
Nature 552(7684): 239-243. - Shahbazi MN., Jedrusik A., Vuoristo S., Recher G., Hupalowska A., Bolton V., Fogarty NM., Campbell A., Devito LG., Ilic D., Khalaf Y., Niakan KK., Fishel S., Zernicka-Goetz M. (2016)
Self-organization of the human embryo in the absence of maternal tissues.
Nat Cell Biol. 18(6): 700-708.
Group Members
- Viviane de Souza Rosa
- Miguel Ángel Ortiz Salazar
- Rina Sakata
- Nanami Satoh
- Luca Schwarz
- Anfu Wang